11 Jun Successful Implant of Barostim Baroreflex Activation Therapy for the Treatment of Heart Failure Symptoms
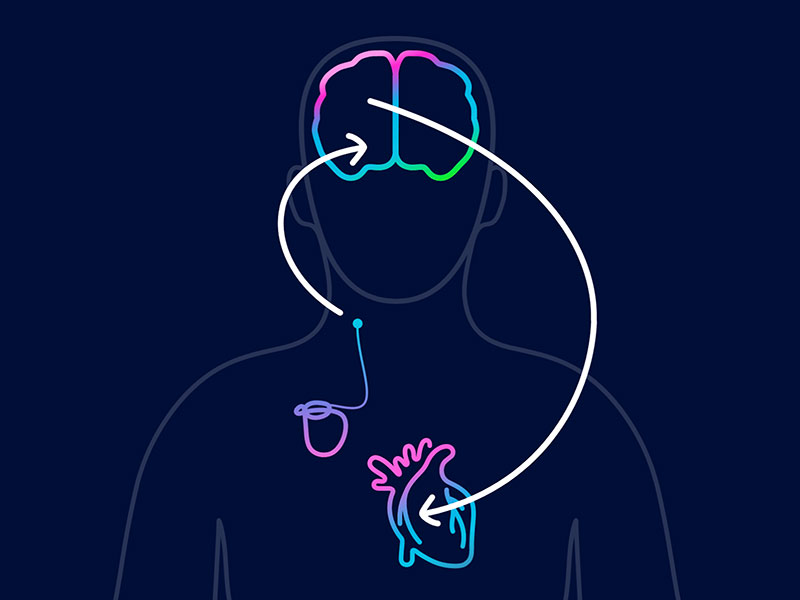
Andover, KS – KANSAS MEDICAL CENTER has announced the hospital’s first successful implantation of Barostim™ Baroreflex Activation Therapy, the world’s first FDA–approved heart failure (HF) device to use neuromodulation – the power of the brain and nervous system – to improve the symptoms of patients with systolic heart failure. This advanced, award–winning therapy was designed to treat HF patients who have had little to no success with other proven treatment options.
Unlike other HF treatment options, Barostim does not touch the heart and utilizes an electrode that lies on the patient’s carotid artery. The electrical impulses that are sent from the neuromodulation device inform the brain of the heart’s condition, allowing the brain to improve the function of the heart. Over time, the organ will regain strength, with the symptoms of HF lessening, enabling patients to return to normal activity. This unique technology is customizable to meet each patient’s individual therapy needs and offers the potential to improve quality of life and reduce health risks associated with HF, including heart and kidney disease, stroke, and death.
Barostim, created my Minneapolis–based medtech company CVRx®, was designated as a Breakthrough Device technology by the FDA, received FDA PMA Approval in 2019 and is now commercially available to reduce the symptoms of HF for patients who are not indicated for CRT and have a left ventricular ejection fraction of 35% or less. Barostim is also the recipient of the Centers for Medicare and Medicaid Services (CMS) Transitional Pass–Through Payment Status (TPT) and inpatient New Technology Add–On Payment (NTAP). The approval of TPT and NTAP for Barostim will help accelerate access to the therapy for the thousands of Medicare patients still suffering from the effects of heart failure.

